MR-306 series syringe pumps have been registered in Germany
On January 7, 2020, 306 series syringe pumps obtained CE certification, which is the first product of MRH that has passed the CE certification. In order to enable the products to be sold to more countries, MRH started the registration in Germany immediately. Despite the spread of COVID-19 in Europe, the registration progress was actively promoted, and the registration was successfully completed on August 5. This marks another solid step in the development of MRH products into the European market.
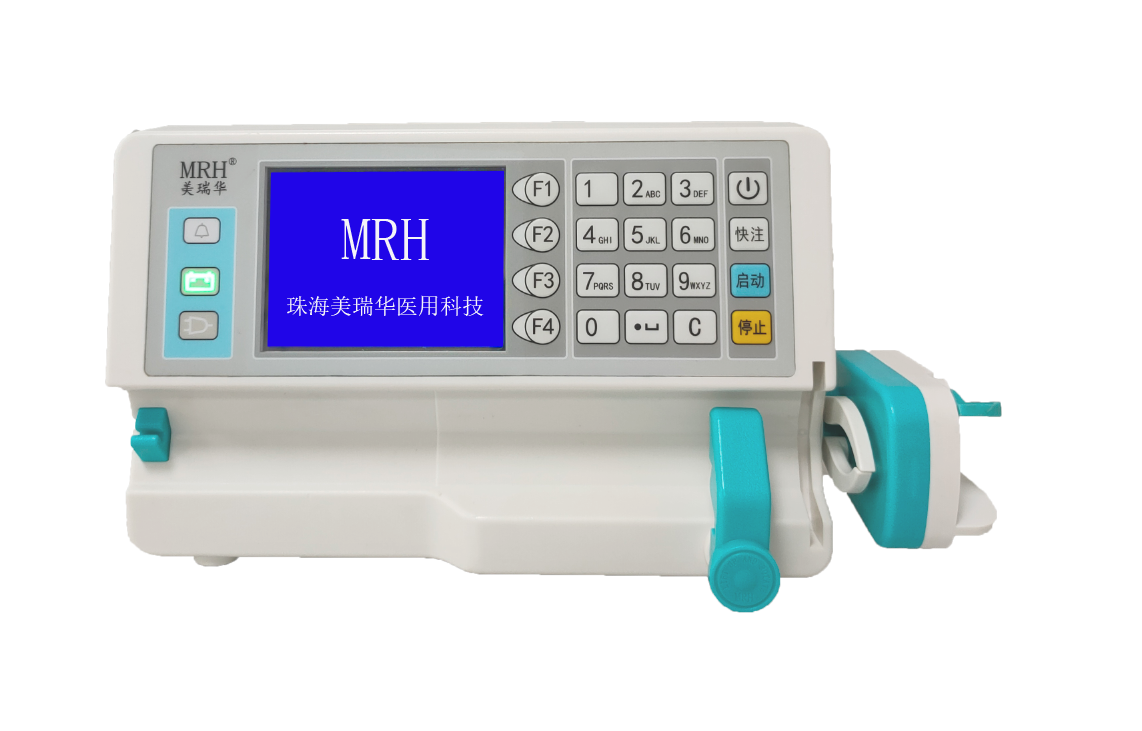
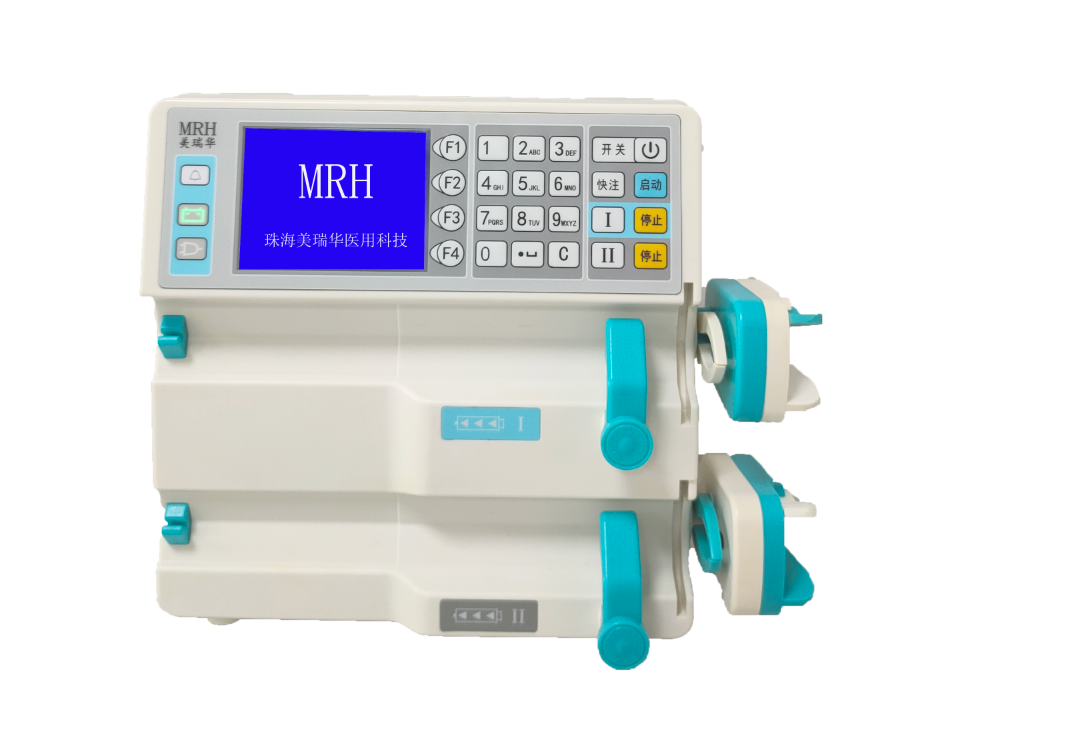
MRH has been focusing on precise and safe infusion pump products for medical use, and is committed to building a prestigious medical instrument brand at home and abroad. In the future, MRH will continue to perform well.